Tap Density Test Apparatus
- Product Description
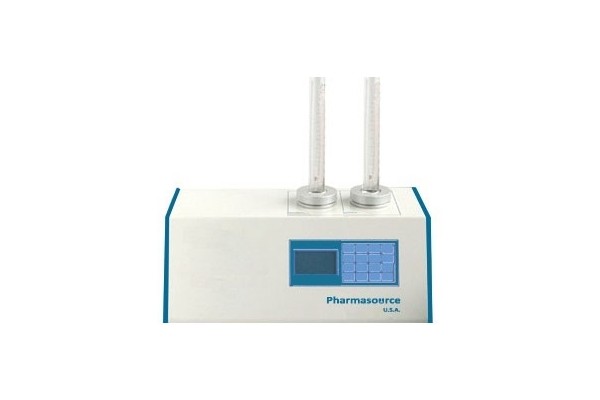
PTD 302 / 302P Two Cylinders Tap Density Test Apparatus
Product Descriptions :
The Pharmasource tap density test apparatus with specifically designed for USP method I and II applications and perfect instruments for measuring of tapping density in the pharmaceutical powders, granules, pellets and bulk testing compliance with as per USP, BP, EP, JP, CP regulations and others international pharmacopoeias specifications and guidelines.
Our PTD 302 series dual cylinders tap density testers with accurate measurements functions for testing of bulk density in powders and granules according to the international pharmacopoeias standard. The apparatus can operate manual, automatic or continuous and samples tested by individually with manually place on the sample cylinders. The tester with two changeable glass cylinders 100ml, 250ml structures for easy to samples place, tested, powders clean and place the new sample for test. The two measuring cylinders can measure two samples at the same time and automated overload measurements protection with safety systems and smooth motorized samples test techniques with low power consumption.
The instruments assemble with microprocessor controls and complete monitoring systems for precise tap density testing with accurate results. The automatic self-checking systems for any error and powerful drive head with high precision stepper motor for vibration setting USP method I and II. The precision powerful vibration motor with stable shaking motion, low noise, long service life and auto calibration by external calibrated meters. The setting parameter of tapping speed, timer, vibrations number and resetting functions by LCD keypad control panel with user friendly interface, easy to operations, fast replacement and constant adjust cylinders position.The apparatus with two measurements systems USP method I for 300 tap and USP method II for 250 tap per minute and automated rotation of cylinders during the test process. The precision speed controller and integrated sensors continuously transfer the information to real-time display monitor of testing results and reliable performance. After the test methods finished instruments automatically calculation of measurements report, times, vibrations frequency and test data prints.
Typical Applications :
The Pharmasource tap density test apparatus applications for USP method I and II and perfect instruments for regular use in the field of pharmaceutical testing, quality control, research and developments, education, industrial analysis and measuring of tapping density in the powders, granules, pellets and bulk testing as per compliance with USP, BP, EP, JP, CP regulations and others international pharmacopoeias requirements and specifications.:
:
PTD 302 [ 302P with data printer ]
:
2 positions
:
100ml and 250ml with sample level marked
:
Two clear view glass cylinders with supporting holders
:
300 tap per minute for USP method I
250 tap per minute for USP method II:
14mm for USP method I
3mm for USP method II:
±0.5%
:
Automatic, manual or continuous
:
Vibration controlled precision motor
:
0-9:59:59 [ adjustable hours, minutes and seconds ]
:
0-99999 times
:
External calibrated meters
:
LCD with actual working status
:
Tapping speed, timer, vibrations number and resetting functions
:
Constant adjust cylinders centering position
:
<60dB
:
Continuous 23:59 hours
:
Temperature 5~38°C and Humidity 80%
:
Automatic self-checking systems for any error
:
Built-in data printer [option]
:
Measurements report, times, vibrations frequency and test data
:
GLP, GMP by international pharmacopoeias standard and requirements
:
Overload protection with safety systems
:
220V, 50Hz operations [110V, 60Hz option]
Features & Advantages :
◊ Compliance with USP, BP, EP, JP, CP and others pharmacopoeias specifications and requirements.
◊ Specific design, advanced technology and reliable performance.◊ Test supported for USP method I and II with 100ml and 250ml cylinders.
◊ Microprocessor controls systems for precise testing with accurate results.
◊ Two cylinders structures for easy to samples place, tested and powders clean.
◊ LCD screen with actual working status.◊ Automatic test data calculations, analysis and results measurements.
◊ Vibration setting, timer and start-stop by front keypad control panel.
◊ Precision speed controller with integrated sensor.
◊ Easy to calibration by external calibrated meters.
◊ Individually samples tested by manually place on the sample cylinders.
◊ Auto adjusting centering cylinders position and fast replacement.
◊ Motorized smooth samples test techniques and low power consumption.◊ Automatic self-checking systems for any error.
◊ Overload measurements protection with safety systems.
◊ Capability of continuous operations 23:59 hours.
◊ Reset programs and automatically save settings parameter.
◊ User friendly interface and easy to operations.◊ Compliance with GLP, GMP by international pharmacopoeias standard and requirements.
Options and Accessories :
Pharmasource 100ml clear view glass cylinder with sample level marked
250ml clear view glass cylinder
Supporting holders, screws and knobs
Calibrated meters with certificates
User Documentations :
Operational Manuals [in English]
IQ > Installation Qualification
OQ > Operational Qualification
PQ > Performance Qualification

